Who is Sanguina?
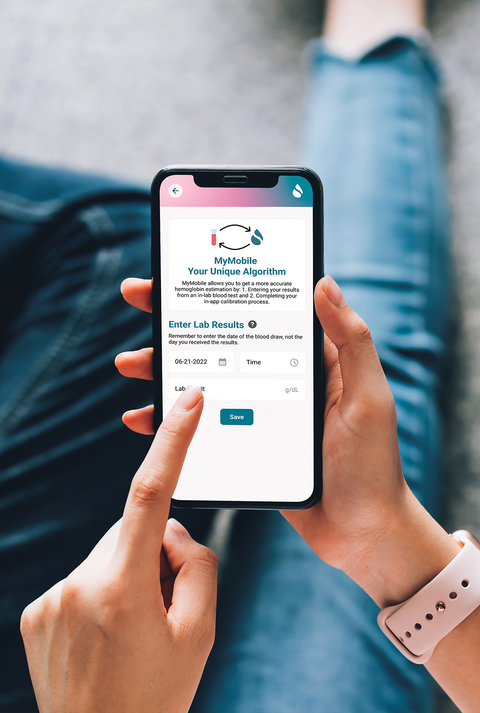
Our CEO and founder Erika, and her business partner, Rob, are just two of more than 1.62 billion people worldwide who struggle with anemia, the most common blood disorder.
Their personal battles inspired them to develop affordable and accessible products for regular anemia screening, so people like them could be proactive about their health.